
Rats are used in biological and medical studies. This is because they are very close relatives to humans on the tree of evolution. If we can solve a health problem using a rat, there is a good chance we can use a similar method to cure sick people. If we test a new product on a rat and the rat gets sick or dies, we know that this product might be dangerous to humans too. Modern medical studies use rats as living models of human health and disease. It is the ability of rats as a model to predict the human response accurately that makes them our sacrificial animal of choice.
Another reason we use rats in research is that, historically, rats have been considered pests and even vectors for disease. Many people are afraid of rats and it is not unusual to see rattraps for sale at your local hardware store. At the same time, there are many people who keep rats as pets. They can be a loving and intelligent companion species and they are sold in many pet stores. What is for some an animal species that should be exterminated is for others a friendly and familiar cousin deserving care and empathetic communication. It is confusing, isn’t it?
Transgenic rats are different than wild type rats. Transgenic rats are rats that have foreign DNA inserted into their genome. This means one or more genes from a non-rat organism (i.e. human, fish, plant or jellyfish) has been added, through some tricks of modern molecular biology, to every one of a trangenic rat’s cells. Transgenic rats are walking around with non-rat expressible molecules in their bodies, minds and even in the cells that go on to make their children. Sometimes referred to as hybrids, cyborgs or chimeras, transgenic organisms are an interspecies mix of DNA, a targeted collage of two or more organisms. The most important thing to remember is that their alteration is permanent and inheritable. This means that their kids and their grandkids with have the same difference that they do.
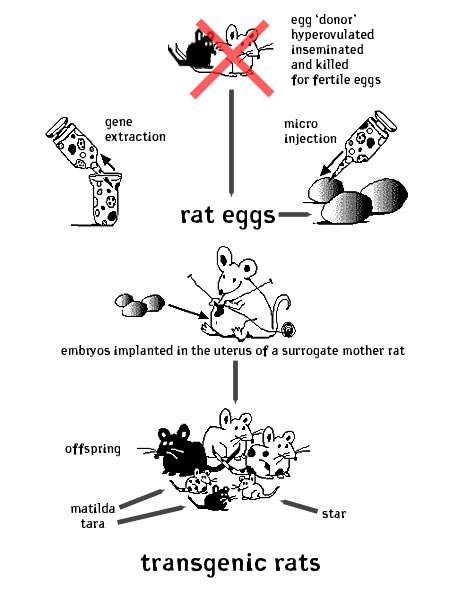
The process of ‘creating’ a transgenic rat is technical and sort of gross. First, the scientist or breeder chooses a gene. This gene is isolated and many copies of it are made using the techniques of molecular biology (PCR, etc.) Then hormone injections are given to the egg-supplying (donor) rat [mom(1)] for hyper-ovulation. She releases many, many eggs from her ovaries. She spends her last night with a frisky male inseminator. Then she is killed and her fallopian tubes are cut open and the fertile rat eggs are suctioned out. These eggs are viewed closely under a microscope and a very small needle is used to inject them with the gene of choice. Then, hormone injections are given to one or more surrogate 'uterus' donors [moms(2)] to simulate a psuedopregnant state. The embryos that tested positive for having taken up the new gene are implanted into the surrogate uterus of the [moms(2)] rats. The embryos that don’t test positive are destroyed. Any rats that are born not expressing the gene of choice are killed after birth. Any rats that do have the transgene in their genomes are bred with each other and then bred with their sons and daughters to secure the continued transmission of the gene. matilda, tara and star barbie are retired breeders who were tested and guaranteed to have a stable transgenic heritage. They have had 1-3 litters of transgenic rats, all of whom have been sold to labs for the study of various diseases related to their genetic alteration.
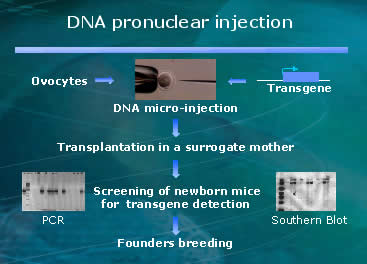
Genetically manipulated animals, like HLA-B27 humanized rat, are considered promising tools to decode physiological processes and cure diseased metabolisms. Sometimes animal models are not the best mirrors of human health. Rats and humans are similar but if we can make rats more similar to humans then we can increase predictability. So, in the name of progress and a faster drug development pipeline, molecular biologists in the medical field are creating human-rat hybrids as disease models.
Specialists in agriculture and animal husbandry have other goals than human health. Some want more yield for more profit, others want disease resistant organisms to erase fears of chaotic loss. There are even some pet producers who are using transgenic techniques to create aesthetic differences as a way to make newer, more seductive, cuter or stranger companion animals. And, some animals are used as industrial factories for producing rare metabolites. After ornate molecular tweaking organisms known as workhorses are bred to transgenically produce products as an excreted fluid, products like pharmaceuticals or other expensive goo. These products are hard to make in a chemistry lab but can be produced in large enough amounts inside a body that a company can live off these special transgenic bodies and their body fluids. These animals are considered to be alive only as appliances or production facilities solely for manufacturing.
It is the medical applications that carry the bulk of the reasoning for the application of these technologies. Although medical science is a ‘for profit’ venture worldwide, there is a certain amount of awe for doctors and scientists who dedicate their life work and somewhat cryptic brainpower to advances in curing human ills. Although far from a cure all, modern medical science has helped many people live longer and better lives. Nonetheless, It is important to realize the other uses and forces driving novel transgenesis as a breeding process for commercially engineered or, some would say, force-evolved processing units to manufacture proteins for the global market.
These rats are named Matilda, Tara and Star. These are all transgenic (gene
transfer) rats, HLA-B27 transgenic rats to be precise, exhibiting a phenotype
similar to humans suffering B27 related rheumatic disorders. They have been
microinjected with human DNA that sets them up for a precondition to be autoimmune
challenged. The injection of human genetic material occurs in the pronucleus
of mouse embryo, and it is passed generation to generation ever after. They
are prone to develop diseases like reactive arthritis, psoriasis inflammatory
bowel disease, and other things. They are developed for pharmaceutical research
studies in systemic inflammation. Matilda, Tara and Star are also retired
breeders, meaning they were used to give birth to baby rats that carried their
added gene. They came from a laboratory that breeds such rats (and mice too)
and sells them to researchers. They were created as a disease model
by a group of scientists. One of these scientists, Dr. Joel Taurog,
was kind enough to let me interview him. You can read the transcript
of the interview here: the barbie’s origin story.
DNA: Deoxyribonucleic acid; Molecule that encodes genetic information; main
component of the chromosomes; DNA is a double-stranded helix held together
by bonds between pairs of nucleotides.
Fertilized rat egg: embryo at "one cell" stage of development
Gene: DNA sequence encoding one particular protein
Genome: genetic heritage from an individual or species; the set of genes that
ensures transmission of hereditary characteristics
Germ cells: sperm and egg cells, and their precursors. Germ cells are haploid
(only one set of chromosomes, while the other somatic cells have two copies)
and transmit the genetic characteristics to the lineage
Insertion: genetic mutation by introduction of one or more nucleotides in
a DNA sequence
Knock-in: describes a mouse in which one particular gene sequence has been
replaced by another
Knock-out: describes a mouse in which one particular gene sequence has been
modified to block gene expression
Micro-injection: technology used in the laboratory to introduce a transgene
into fertilized rat eggs
Mutation: change in nucleotide sequence of DNA
Predictability: property of a research system (e.g., an animal model) to mimic
human physiology. A model of high predictability provides researchers with
results that are closer to those they would obtain directly if they could
perform the experiments in humans.
Transgenic: organism (mouse) whose genome has been altered by the inclusion
of foreign genetic material
Vector: tool (particular DNA sequence) permitting the transport of foreign
or modified DNA
Brinster, R. (1974). The effect of cells transferred into mouse blastocyst
on subsequent development. J. Exp. Med.:1049-1056.
Donnelly, S., McCarthy, C.R. and Singleton, R. Jr. (1994). The Brave new World
of Animal Biotechnology, Special Supplement, Hastings Center Report.
Federation of European Laboratory Animal Science Associations (FELASA) September
1992, revised February 1995. Transgenic Animals - Derivation, Welfare, Use
and Protection.
Gordon, J.W. and Ruddle, F.H. (1981). Integration and stable germ line transformation
of genes injected into mouse pronuclei. Science 214:1244-1246
Gossler, A. et al. (1986). Transgenesis by means of blastocyst-derived embryonic
stem cell line. Proc. Natl. Acad. Sci. 83:9065-9069.
Jaenisch, R. (1976). Germ line integration and Mendelian transmission of the
exogenous Moloney leukemia virus. Proc. Natl. Acad. Sci. 73:1260-1264.
Moore, C.J. and Mepham, T.B. (1995). Transgenesis and animal welfare. ATLA
23:380-397.
US Congress, Office of Technology Assessment (1989). New Developments in Biotechnology:
Patenting Life. Special Report OTA-BA-370. 3pp. Washington DC: US Printing
Office.
Read Kathy High's interview with Dr. Joel Taurog, one of the originators of the HLA-B27 transgenic rat line:
The term transgenic animal refers to an animal in which there has been a deliberate modification of the genome - the material responsible for inherited characteristics - in contrast to spontaneous mutation.
-FELASA September 1992, revised February 1995

|

|
|
|||
| |
|
|
|
||
| |
|
||||
| |
|
||||
| |
|
||||
| |
|
||||
| |
|
|
|
||
| |
|
||||
 |
|
||||
|
|
|
||||
|
|
|
|
|
|
|